
Thinksmart
Lead With Thinksmart
EXT 220 MED | 3D SYSTEM
Extrusion 3D printer specifically designed for the additive manufacturing of implants
The EXT 220 MED with its filament-based extrusion technology enables printing of medical implants and instruments using high performance polymers, including PEEK, resomers and Radel® PPSU. It is the only platform with an integrated clean room and temperature controls to enable high-quality device production.

The platform delivers rapid, reproducible printing for a broad range of applications and is validated by leading hospitals and device manufacturers worldwide. Additional benefits of the EXT 220 MED 3D printer include:
1. Optimized for operating in clean rooms
2. Fully controllable build chamber heating
3. Validated to fulfill ASTM F2026 standards
4. Excellent accuracy due to delta kinematics
5. Laminar airflow enabling homogenous temperature distribution
6. Adaptive local temperature management
FDA clears first 3D-printed PEEK cranial implant
Discover the VSP® PEEK Cranial Implant. This patient-specific solution offers a precise fit and reliable performance for cranioplasty procedures to restore defects in the skull. See how the EXT 220 MED enabled this innovation.

Elevate Quality with Temperature Management
The EXT 220 MED is equipped with a global laminar air flow, which allows users to heat the build chamber homogenously up to 250 degrees Celsius. The additional local air flow helps improve the mechanical properties of devices. The platform is designed to control temperatures during production to enable high-quality, 3D-printed medical devices produced using thermogenic polymers, like PEEK and Radel® PPSU.

Fully controllable build chamber heating
Heat up to 250 degrees Celsius, prevent device warping, increase layer adhesion, and adapt build chamber temperature to suit specific polymers

Laminar airflow enabling homogenous temperature distribution
Achieve excellent reproducibility with no temperature gradient within the build part

Adaptive local temperature management
Adjust local cooling for each strand and layer individually, heat up to further increase layer adhesion, reduce the need for post-processing, and simplify removal of support structures
Make the Build Chamber a Clean Room
With the help of the integrated filter system, the build chamber can be turned into a clean room to avert contamination. What’s more, the 3D printer is “clean room ready” for production in existing sterile environments and meets medical standards.


Optimized for operating in clean rooms
1. Integrate the EXT 220 MED platform into standard medical production environments
2. Receive support to pass medical qualification processes (IQ,OQ,PQ)
3. Use the extractor hood to connect the printer to your clean room air exhaust

Includes a filter system
1. Keep particles out and prevent contamination
2. Fulfill ISO class 7 clean room requirements according to DIN EN ISO 14644

Features advanced contamination prevention
1. Eliminate the risk of contamination of filament and final parts
2. Simplify cleaning and maintenance with smart design such as touch-to-open hinges

Monitor Processes and Assure Quality
Besides monitoring all relevant process parameters, the industrial-grade control system and software used in the EXT 220 MED make process analytics and documentation feasible even in highly regulated industries.
Sample Application
The EXT 220 MED with its filament-based extrusion technology enables printing of medical implants and instruments using high performance polymers, including PEEK, resomers and Radel® PPSU. It is the only platform with an integrated clean room and temperature controls to enable high-quality device production.



Sample Applications

Craniomaxillofacial Implants
Material: PEEK
Patient-specific cranial and orbital plates are 3D printed using a complete design-to-delivery workflow. Manufacturing time is as little as two days from scan to surgery.

Interbody Fusion Devices
Material: PEEK BCP
PEEK filled with BCP or HA-combined with integrated lattice structures-is intended to support cell attachment and proliferation.

Facial Implants
Material: PEEK
Patient-specific devices compatible with standard fixation systems. Printed in 30-60 mins.

Orbital Floor Implants
Material: PEEK Barium Sulphate
3 Enhancing PEEK with Barium Sulphate ensures visibility in imaging technology.
Compatible Materials for the EXT 220 MED Printer

PEEK Material
High-performance, biocompatible polymer ideal for medical implants
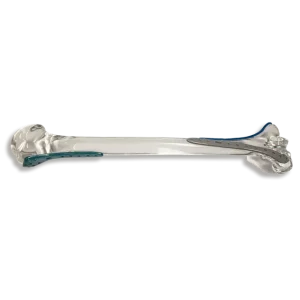
Radel PPSU (Polyphenylsulfone)
High impact strength polymer ideal for sterilizable medical instruments
View All Medical Materials
EXT 220 MED enables patient-specific
PEEK implants at the point-of-care
Discover the life-changing possibilities of 3D printing through
Salzburg University Hospital’s significant medical milestone: its
first implantation of a 3D printed PEEK cranial plate.
Liên hệ trụ sở Thinksmart gần nhất
⊙ Thinksmart Hồ Chí Minh: Số 5, Đường số 4, Khang An, P. Phú Hữu, Tp. Thủ Đức, Tp. Hồ Chí Minh.
0286 2715 968 ( Tư vấn công nghệ – Mr. Danh )
0968 968 468 ( Tư vấn thương mại sản phẩm – Anh Thủy )
⊙ Mail: info@thinksmart.com.vn
⊙ Thinksmart Hà Nội: Số 7, Ngõ 6, Phố Cửu Việt, TT. Trâu Quỳ, H. Gia Lâm, Hà Nội
036 549 8888 ( GĐ Hà Nội: Anh Tùng – Tư vấn công nghệ )
⊙ Mail: Tung_tran@thinksmart.com.vn
⊙ Thinksmart Hải Phòng: Số 42 Lê Thánh Tông, Máy Chai, Ngô Quyền, Hải Phòng.
0973 788 289 ( Tư vấn công nghệ )
⊙ Mail: info@thinksmart.com.vn
⊙ Thinksmart Đà Nẵng: 44 đường Trần Lựu, phường Hòa Xuân, Quận Cẩm Lệ, TP Đà Nẵng
097 478 2411( Anh Trung – Tư Vấn Công Nghệ )
⊙ Mail: Trung_mai@thinksmart.com.vn
⊙ Thinksmart Huế: Đường số 79/107 Phùng Hưng, TP Huế, Tỉnh Thừa Thiên Huế.
090 434 23 94 ( Anh Huy- Tư vấn công nghệ )
⊙ Mail: info@thinksmart.com.vn
⊙ Thinksmart Vũng Tàu: số 42, Khu Phố Hoàng Giao, TT. Ngãi Giao, H. Châu Đức, Bà Rịa Vũng Tàu.
0968 968 468 ( Anh Thủy – Tư vấn công nghệ )
⊙ Mail: info@thinksmart.com.vn
⊙ Thinksmart Cần Thơ: Tòa nhà Korea Vietnam Incubator Park (KVIP), đường số 8, KCN Trà Nóc 2, P. Phước Thới, Q. Ô Môn, TP. Cần Thơ.
0787 896 032 ( GĐ Cần Thơ: Anh Sơn – Tư vấn công nghệ )
⊙ Mail: son_tran@thinksmart.com.vn
⊙ Thinksmart Texas, US: 12747 Justin Trail Houston, TX 77070, US.
+1 714 312 9207 ( Technology advisory )
⊙ Mail: info@thinksmart.com.vn
⊙ Xưởng chế tạo: Số 87, KĐT Vinhome Oceanpark 2, Văn Giang, Hưng Yên, Hà Nội
Thinksmart Hân Hạnh Phục Vụ
⊙ Thương mại : Máy in 3D Resin để bàn Formlabs, Máy in 3D SLA Cao cấp , Máy 3D công nghiệp, Máy quét 3D / Scan 3D Photogrammetry công nghiệp, Máy CNC cỡ nhỏ,Phần mềm Phân tích tính toán & Mô phỏng Altair, Phần mềm Geomagic Design X & Geomagic Control X, gói giải pháp công nghiệp.
⊙ Dịch vụ Trọn gói : Quét 3D / Scan 3D + Photogrammetry & Thiết kế ngược, Thiết kế kiểu dáng & kết cấu công nghiệp, RTM, Chassis, Frame, Body, IP, Trim,… Phân tích tính toán & Mô phỏng Altair, In 3D cao cấp, CNC Robot, Mockup Buck, 3D Inspecting/QC/Test, Chế tạo.
⊙ Cung cấp gói giải pháp cho mảng: Ô tô, Y tế, Hàng không, Công nghiệp và Dịch vụ R&D,…
Copyright © 2019 Thinksmart Inc. All rights reserved..





